UK govt invests £10m in tech to destroy cancers and predict disease
- 14 February 2024

The UK government has made a multi-million pound investment in potential breakthrough medical devices.
As part of a £10 million funding package for boosting access to medical technology, eight innovative tech companies will be supported to bring their devices to market. It could help transform the way clinicians treat some of the biggest causes of death and disability in the UK.
One device, by HistoSonics, aims to identify and destroy liver cancer tumours using focused ultrasound waves. These waves break down tumours without damaging healthy tissue, offering a safer alternative to radiotherapy and other high intensity treatments. It could improve quality of life for many patients going through treatment – reducing hospital visits and post procedure complications and making pain management easier.
Another company is developing a blood test for Alzheimer’s Disease, which means patients could be identified and treated earlier. Roche Diagnostics Ltd has developed the Amyloid Plasma Panel – a blood test that could help clinicians decide if patients with cognitive impairment should undergo tests or imaging to confirm Alzheimer’s Disease.
A second portable blood test, from Up Front Diagnostics, could help paramedics identify stroke patients more quickly. Currently, ambulance workers can’t recognise a patient with a blood clot blocking the flow of blood and oxygen to their brain, who would require urgent treatment at stroke centres rather than at local hospitals.
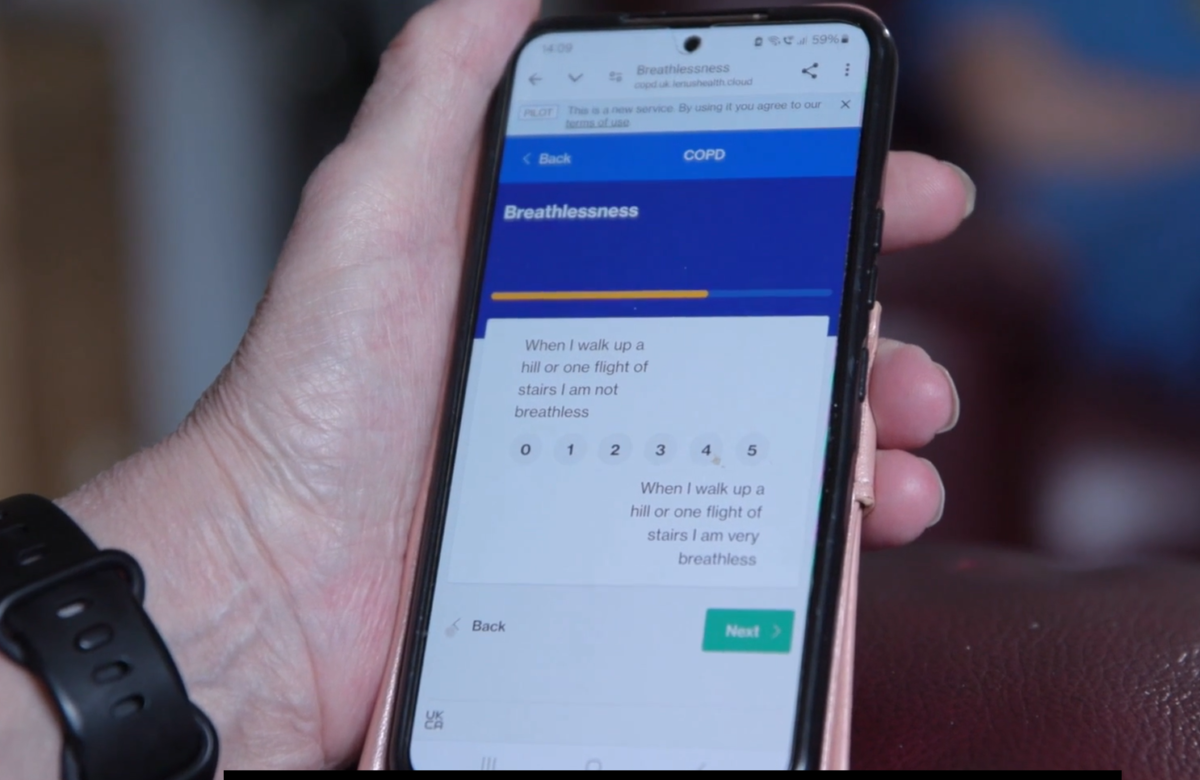
Lenus Health Ltd is using AI to predict patients at risk of hospitalisation for Chronic Obstructive Pulmonary Disease, which causes the airways to become narrow and damaged, resulting in breathing difficulties. The company collects data from wearable devices, sensors and apps and uses AI to predict which patients are at greater risk of hospital admissions.
An additional device aims to reduce inequalities in the field of lung health. Oximeters – devices clipped over the end of a fingertip – are used widely at hospitals and at home to assess how well the lungs and circulatory system are working. However, research suggests this technology may not accurately detect falling oxygen levels in people with darker skin tones.
EarSwitch has produced a device which detects oxygen levels from the inner ear-canal instead, which is not pigmented irrespective of the person’s skin colour. It could offer better quality readings and a more innovative approach to oxygen level monitoring.
Other technologies set to benefit from a share of the funding include: multiple sclerosis fatigue app Avegen Ltd, which is developing a new smartphone app that delivers exercises, cognitive behaviour therapy and targeted physical activity; 52 North Health, which has a new device to allow chemotherapy patients to self-test at home for the potentially life-threatening condition neutropenic sepsis; and Presymptom Health Ltd, an algorithm infection predictor for Systemic Inflammatory Response Syndrome (SIRS), a life-threatening medical condition caused by the body’s overwhelming response to infection or inflammation.
Package part of long-term plan
Today’s announcement is part of the government’s long-term plan to ensure the NHS and its patients can get quicker access to new groundbreaking technologies. It follows the unveiling last year of the Department of Health and Social Care’s blueprint for boosting NHS medtech and turning innovation into real benefits for society.
Responding to the announcement, Health Minister Andrew Stephenson said: “NHS staff need access to the latest technology to deliver the highest quality care for patients and cut waiting lists – one of our top five priorities.
“These cutting-edge technologies could help thousands of patients with a range of conditions, including cancer, stroke, and Alzheimer’s, while easing pressure on our hospitals and reducing healthcare inequalities.”
Dr Marc Bailey, Medicines and Healthcare products Regulatory Agency chief science and innovation officer, said: “This is designed to explore how support from the regulator, UK health technology organisations and NHS bodies can accelerate the development of transformative medical devices from their initial proof of concept through to uptake in the NHS.
“The pilot criteria prioritises patient need in all aspects of decision-making and, by supporting innovative medical technologies, will ease pressure on the healthcare system,” he added.
The funding is part of a new programme called The Innovative Devices Access Pathway (IDAP), which aims to bring state-of-the-art technologies and solutions to the forefront of the NHS. Currently in the pilot stage, the funding will be used to test the new technologies for use on a large scale as quickly as possible.
The programme is run by the Medicines and Healthcare products Regulatory Agency (MHRA), The National Institute for Health and Care Excellence (NICE), NHS England, Health Technology Wales, and Scottish Health Technology Group. They will be providing tailored, intensive advice on regulatory approval, health tech assessments and access to the NHS.